fish
In the Field
27/05/15 10:15
The semester is over! Tralalalalalalaaaaaa!!
Hey, I love teaching. And there are parts of my job that I love. But most of those parts are concentrated in the few days a year when I’m working, but not in my building. Right now none of my projects are in active need of field sampling or monitoring or measuring anything, so I am living vicariously through my students. Christine led an expedition out to Sapelo last week, where the UGA Marine Institute put us up in a house on the marsh for 5 days of finding horseshoe crabs (for their Chelonibia epibionts - Christine’s dissertation beast), exploring the marsh, teaching my 8-year-old the taxonomy of fiddler crabs, a little beach time, and a lot of writing and excellent together time, all of it much needed.








It goes without saying I have about 200 more pictures as good as those or better. We got our quarry, maybe 1-2 more field trips for Christine on her way to doctorhood! We were all sad to leave Sapelo (the loss of a day in cleaning, driving, unpacking, exhaustion, back to building), but quickly got to look forward to the next trip - south central Georgia for a day on a river with Katie Bockrath and Mary Freeman’s fish crew, who helped get us a second round of mussel hosts collected on a beautiful day before a big storm. The timing is everything - water temperature, how high the stream is running, whether it is storming of course, whether we can round up a truck on short notice (not to mention a crew) - and this was almost effortless for once!






Thanks to everybody in the lab and otherwise who helped on these trips, in any way - these are the days I most feel like a biologist.
Hey, I love teaching. And there are parts of my job that I love. But most of those parts are concentrated in the few days a year when I’m working, but not in my building. Right now none of my projects are in active need of field sampling or monitoring or measuring anything, so I am living vicariously through my students. Christine led an expedition out to Sapelo last week, where the UGA Marine Institute put us up in a house on the marsh for 5 days of finding horseshoe crabs (for their Chelonibia epibionts - Christine’s dissertation beast), exploring the marsh, teaching my 8-year-old the taxonomy of fiddler crabs, a little beach time, and a lot of writing and excellent together time, all of it much needed.








It goes without saying I have about 200 more pictures as good as those or better. We got our quarry, maybe 1-2 more field trips for Christine on her way to doctorhood! We were all sad to leave Sapelo (the loss of a day in cleaning, driving, unpacking, exhaustion, back to building), but quickly got to look forward to the next trip - south central Georgia for a day on a river with Katie Bockrath and Mary Freeman’s fish crew, who helped get us a second round of mussel hosts collected on a beautiful day before a big storm. The timing is everything - water temperature, how high the stream is running, whether it is storming of course, whether we can round up a truck on short notice (not to mention a crew) - and this was almost effortless for once!






Thanks to everybody in the lab and otherwise who helped on these trips, in any way - these are the days I most feel like a biologist.
Yellowfins or Greenheads?
10/07/13 10:32
Mark Scott of SC DNR just posted this to YouTube and I wanted to give it a shout-out.
http://youtu.be/2vJoEraw4QY
Very nice underwater footage of the Middle Saluda River, in the Santee Basin of South Carolina. Whenever you drive over a bridge and look down at the brown waters of southern rivers, don’t think for a minute there isn’t a really interesting aquatic world beneath the surface!
The shiners are labeled green head shiners in this video, Notropis chlorocephalus. Until very recently they were considered part of the yellowfins that I have worked on (N. lutipinnis), but Mollie Cashner has been doing phenotypic and genetic work to show that there is more going on in terms of the history of these fish and these drainages.
http://youtu.be/2vJoEraw4QY
Very nice underwater footage of the Middle Saluda River, in the Santee Basin of South Carolina. Whenever you drive over a bridge and look down at the brown waters of southern rivers, don’t think for a minute there isn’t a really interesting aquatic world beneath the surface!
The shiners are labeled green head shiners in this video, Notropis chlorocephalus. Until very recently they were considered part of the yellowfins that I have worked on (N. lutipinnis), but Mollie Cashner has been doing phenotypic and genetic work to show that there is more going on in terms of the history of these fish and these drainages.
Still Have Mojo
26/02/11 14:13
I love teaching the Evolution lab class at Georgia. I really do. But this class has led to more sleepless nights over the past 6 years than any other; why? Because maintaining a good flow of information, education, and fun in a lab class is tricky. And while failure is a large part of science, it doesn’t play well when you have a limited number of opportunities to teach and a number of topics you want to teach.
This year GENE 4230 will be repeating Peter Marko’s 2004 work on red snappers. This paper showed that much of what we buy at the store may actually be Lutjanus campechinus; but much of it is not. Fish fillets sold at red snapper prices are sometimes other (rarer) snapper species. Sometimes they aren’t snapper at all, but less valuable fishes. And all of that ambiguity leads questions about how best to manage this threatened stock. Currently, the fishery is closed on our Atlantic coast.
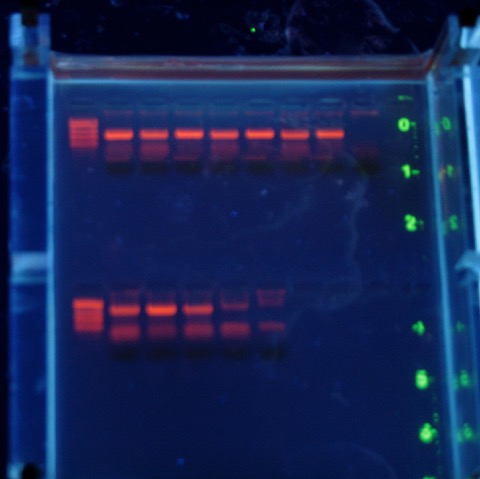
The problem? When I had Kelly double-check my protocol a couple of weeks ago to make sure the PCR would work, it did not. We tried a couple of different protocols, no dice. Finally I decided I needed to jump in and check some things. Long story short? Both our original primer stock, and the last batch of red snapper DNA we had used for this lab 2-3 years ago, were heavily degraded. They had been kept in the aging freezer in the teaching lab, so Genetics department head: there is something that we need to buy this year!
I’m pleased that I could quickly figure out what went wrong, and from the gel above it looks like the lab should work just fine (in case you are wondering, those are the short ‘universal’ fragment primers on top; the CB-12 and -13 primers from Marko’s paper below). There is a little smeariness in the negative control but I also didn’t change my tips in my rush to get this done today (Saturday....and it is 75° outside and sunny). No problem with the top set of reactions, that means I’ll be sleeping much better tonight.